- What is SaMD?
- Why is SaMD Development Challenging?
- The Role of International Standards
- How Is Risk Managed in SaMD Development?
- Practical Tips for SaMD Success
- How Gart Solutions Helps Simplify SaMD Development
- How does the FDA classify SaMD products, and what factors determine their regulation?
- What role does the IMDRF play in global SaMD regulatory frameworks?
- How can developers use FDA guidance documents to navigate compliance for AI-based SaMD?
- Conclusion
- 5 Key Takeaways for SaMD Developers
What is Software as a Medical Device (SaMD)?
Software as a Medical Device (SaMD) is software intended to perform medical functions independently of a physical medical device. This includes mobile apps, AI diagnostic platforms, and cloud-based monitoring systems that diagnose, treat, or prevent diseases.
Let’s explore the essentials of SaMD development, focusing on key concepts, challenges, and compliance with international standards such as IEC 62304 and IEC 82304-1.
What is SaMD?
SaMD refers to software designed to function as a medical device in its own right. SaMD is software intended for medical purposes without being part of a physical device. Examples include mobile health apps, cloud services for diagnostics, and desktop applications for patient monitoring:
- Mobile applications that track health conditions.
- Cloud platforms analyzing medical imaging data.
- Standalone desktop programs offering therapeutic recommendations.
Why is SaMD Development Challenging?
1. Software-Specific Risks
Unlike hardware devices, software inherently includes potential bugs that may emerge unpredictably, potentially impacting patient safety. For instance, a critical failure in SaMD could result in life-threatening situations. Unlike mechanical devices, software failures can be unpredictable and difficult to manage post-deployment.
2. No Manufacturing Controls
SaMD lacks a traditional manufacturing process, which in hardware provides checks to ensure quality. Instead, SaMD relies entirely on robust development practices, continuous testing, and quality assurance measures integrated throughout the lifecycle.
The Role of International Standards
Compliance with international standards is essential for regulatory approval and operational excellence in SaMD.
Comparison Table: IEC 62304 vs. IEC 82304-1
| Feature | IEC 62304 | IEC 82304-1 |
|---|---|---|
| Title | Medical Device Software – Software Life Cycle Processes | Health Software – General Requirements for Product Safety |
| Scope | Focuses on the software development lifecycle for medical devices | Focuses on standalone health software products |
| Applies To | Software embedded in or as a medical device | Standalone software used in healthcare (e.g., apps, platforms) |
| Primary Objective | Ensure safe development, maintenance, and support of medical software | Define safety and performance requirements for health software |
| Development Lifecycle Guidance | Yes – defines phases like planning, design, implementation, verification | No – focuses more on product-level requirements |
| Product Validation | Limited – mostly process and documentation focused | Strong emphasis on product-level validation |
| Risk Management | Integrated into each development phase | Aligns with ISO 14971 for health software risk considerations |
| Configuration Management | Required – tracking software versions and changes | Addresses traceability and update processes |
| User Documentation Requirements | Addresses technical documentation needs | Includes detailed guidance for user instructions |
| Usability Considerations | Minimal (handled via IEC 62366-1) | Requires basic usability and safety performance criteria |
| Target Audience | Software engineers, QA teams, regulatory specialists | Product managers, designers, compliance teams |
| Compliance Need | Mandatory for FDA and EU Class II/III medical device software | Increasingly adopted for wellness and digital health platforms |
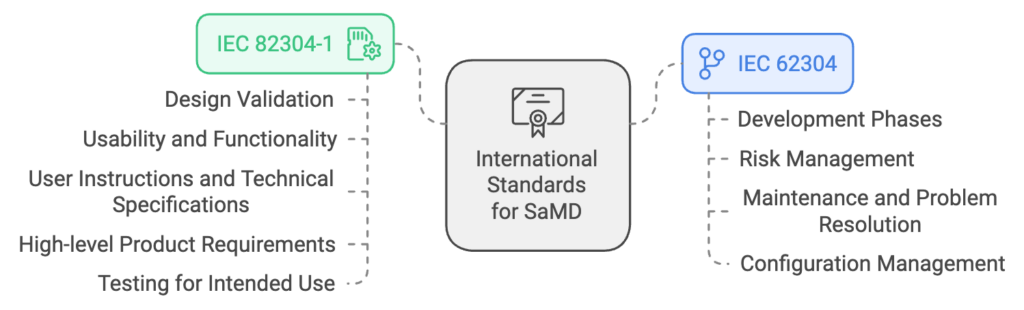
IEC 62304: A Process-Oriented Approach
This standard emphasizes the systematic development of medical device software. It covers essential elements such as:
- Development Phases: Activities and documentation needed for each development phase. Clear requirements for planning, testing, and releasing software.
- Risk Management: Integration of risk assessment at every stage. Incorporates risk controls directly into the development lifecycle.
- Maintenance and Problem Resolution: Ensures ongoing compliance and safety post-release.
- Configuration Management: Ensuring traceability and control over software versions.
IEC 82304-1: Product-Specific Guidance
Primarily for standalone health software, this standard provides guidelines on:
- Design validation
- Ensuring usability and functionality.
- Detailed guidance for creating user instructions and technical specifications.
- High-level product requirements
- Tests to confirm that software meets intended use.
Agile vs. Waterfall: Can Agile Be Used in SaMD Development?
While IEC 62304 is sequential in structure, developers can use agile methods such as scrum to remain flexible. Agile practices like iterative sprints and continuous feedback loops can meet standard requirements while adapting to dynamic project needs.
Though IEC 62304 often aligns with waterfall methodologies, SaMD developers increasingly use agile methods such as scrum. Agile practices allow:
- Testing smaller components in sprints.
- Flexibility to adapt requirements and improve product design.
By integrating these approaches, developers can meet stringent regulatory requirements without stifling innovation.
How Is Risk Managed in SaMD Development?
Risk management in SaMD extends beyond typical engineering concerns. Key components include:
- P1: The probability of hazardous situations occurring.
- P2: The likelihood of harm if the situation occurs.
This two-part approach helps developers focus on reducing harm probability through design and system controls.
P1: Probability of a Hazardous Situation
P1 refers to the likelihood that a hazardous situation will arise during the use of SaMD. For example, a software failure in a medical device that leads to an incorrect diagnosis is a potential hazardous situation.
While some developers assume P1 is always 100% in software risk management, this assumption can be challenged. Real-world examples show that the actual probability depends on specific failure scenarios and their impacts.
P2: Likelihood of Harm
P2 accounts for the probability that a hazardous situation will result in harm to the patient or user. Even if a hazardous situation arises (P1), the harm (P2) may vary depending on the software’s design, user interface, and safety features.
For instance, in a scenario where software misidentifies medication options, P2 is determined by factors like user intervention or alternative safeguards in the system.
Developers use P1 and P2 together to calculate the overall probability of harm. For example:
- A failure in drug selection software could either always choose a dangerous option (high P1 and high P2) or select randomly, spreading risk across options (lower overall harm probability).
- Such distinctions guide the design of safety features and risk controls.
Practical Tips for SaMD Success
Effective configuration management ensures:
- Traceability: Every change is tracked from conception to release.
- Documentation Synchronization: Keeps user manuals and technical specifications aligned with the software.
- Version Control: Tools like Git facilitate efficient branching, merging, and error resolution.
- Combine Lean Practices: Merge design and software releases by embedding regulatory documentation early in development.
- Focus on Usability and Security: Incorporate usability engineering (IEC 62366-1) and cybersecurity (IEC 81001-5-1) as core development activities.
How Gart Solutions Helps Simplify SaMD Development
Developing Software as a Medical Device (SaMD) comes with its share of challenges—from meeting strict regulatory requirements to navigating complex technical demands. Gart Solutions is here to make the process easier. With our blend of expertise and innovative practices, we help SaMD developers stay compliant, streamline workflows, and bring their products to market faster. Here’s how:
Compliance Made Easy
We conduct thorough compliance audits to ensure your SaMD meets critical standards like IEC 62304, IEC 82304-1, and IEC 62366-1. Our team offers practical advice to close compliance gaps and simplify regulatory submissions, whether you’re targeting local or global markets.
Proactive Risk Management
We help integrate risk management directly into your development process, following industry best practices. From identifying risks to designing mitigation strategies, our goal is to enhance safety and minimize potential issues.
Building Strong Health IT Foundations
A solid IT infrastructure is key to successful SaMD. We design systems that are scalable, secure, and seamlessly integrated into healthcare workflows. Plus, we prioritize interoperability with standards like HL7 and FHIR, so your software fits perfectly into the healthcare ecosystem.
Smarter Cloud Migrations
As SaMD increasingly relies on cloud platforms for AI, data storage, and more, we handle secure migrations to keep you compliant with HIPAA, GDPR, and other regulations. We also optimize your cloud setup for better performance, reliability, and cost-efficiency.
Whether you’re building a diagnostic app, therapeutic tool, or any other medical software, we offer tailored software development services. Our agile approach ensures your product is not only compliant but also user-friendly and innovative.
After launch, we assist with performance monitoring, incident reporting, and maintaining regulatory compliance. This keeps your SaMD safe, effective, and ready to meet new standards as they evolve.
At Gart Solutions, we’re more than just a partner—we’re your guide to navigating the complexities of SaMD development. Let us help you create software that doesn’t just meet regulations but redefines how healthcare is delivered.
How does the FDA classify SaMD products, and what factors determine their regulation?
The FDA’s classification system and the key factors that influence the regulation of Software as a Medical Device (SaMD).
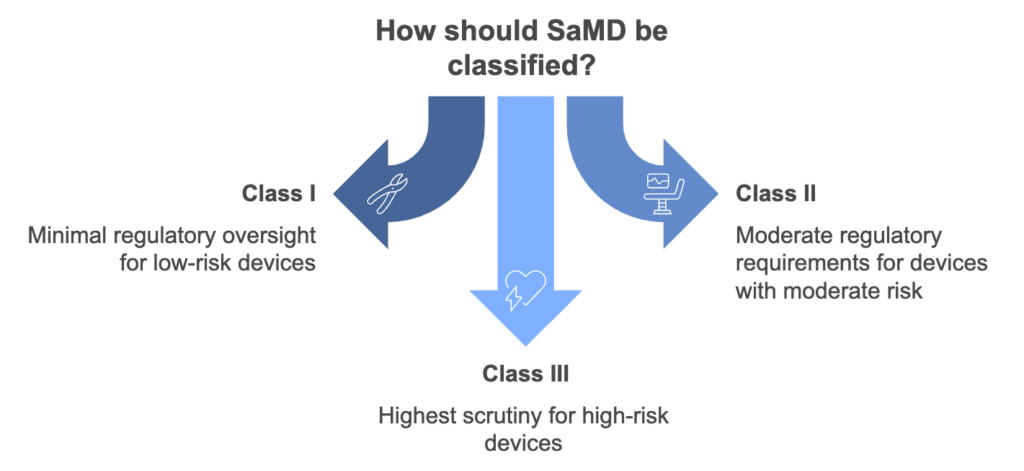
The FDA uses a three-tiered system to classify medical devices based on the risk they pose to patients:
- Class I (Low Risk): Includes devices like tongue depressors and surgical scissors. These are subject to minimal regulatory oversight.
- Class II (Moderate Risk): Examples include cardiac monitors and diagnostic tools, requiring more stringent safety and efficacy evaluations.
- Class III (High Risk): Encompasses devices like implantable defibrillators, with the highest regulatory scrutiny due to potential life-threatening risks.
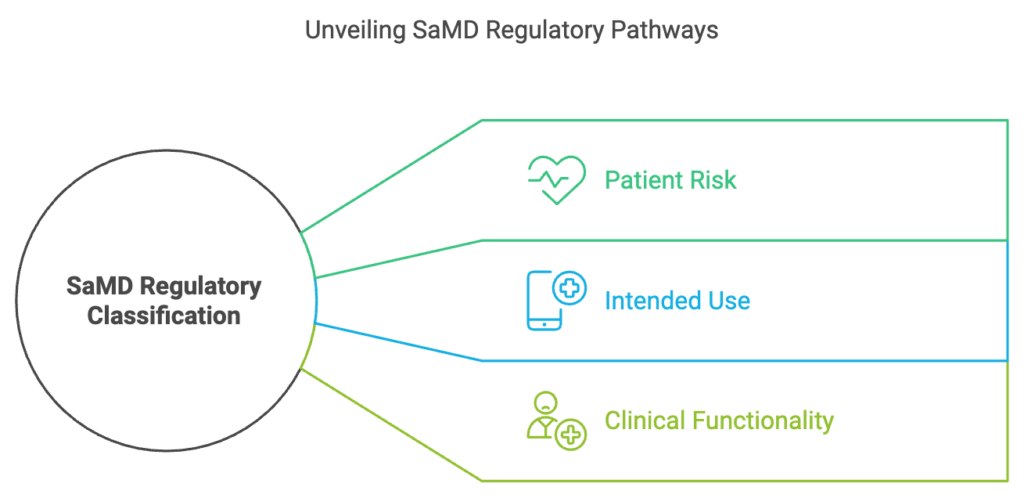
Key Factors in SaMD Classification
Patient Risk
The higher the potential impact on patient safety, the stricter the regulatory requirements. For example, a wellness app monitoring heart rate may not be regulated, whereas a diagnostic tool for cardiovascular diseases likely will be.
Intended Use
The language used to describe a product’s purpose is crucial. If software is marketed for fitness or wellness purposes, it may not be regulated. However, if it claims to diagnose or treat medical conditions, it enters regulated space.
Clinical Functionality
SaMD performing critical functions such as treatment recommendations or automated diagnosis undergoes rigorous assessment. Transparency in decision-making and clinical validation are required for approval.
What role does the IMDRF play in global SaMD regulatory frameworks?
The International Medical Device Regulators Forum (IMDRF) plays a critical role in harmonizing regulatory standards for Software as a Medical Device (SaMD) worldwide.
The IMDRF is a voluntary group of international regulators working together to develop common frameworks and guidelines for medical devices, including SaMD. Their efforts:
- Promote consistent regulatory practices across different countries.
- Simplify market entry for SaMD developers by aligning standards globally.
- Enhance patient safety by sharing information about recalls or incidents across regulatory bodies.
Developing Regulatory Guidelines
IMDRF has established specific working groups for SaMD and AI. These groups provide foundational definitions and requirements for these technologies.
Example: The IMDRF definition of SaMD emphasizes software performing medical functions independently of hardware, clarifying what constitutes a regulated product.
Facilitating Mutual Recognition
IMDRF helps countries recognize audits or approvals conducted by other member regulators, reducing redundancy and streamlining international compliance efforts.
AI-Specific Guidance
The forum recently established a new group focusing on machine learning-derived AI in medical devices, addressing the unique challenges and risks associated with these technologies.
Benefits for SaMD Developers
- Unified guidelines mean less need to adapt products for different regulatory systems.
- Collaboration among regulators ensures that the best practices are shared and implemented globally.
- Clear and consistent rules foster innovation by reducing uncertainty for developers.
The FDA offers a range of guidance documents and tools to assist developers in navigating the regulatory landscape for Software as a Medical Device (SaMD), particularly those incorporating artificial intelligence (AI). Key FDA guidance documents:
- General Guidance for SaMD
Provides foundational requirements for SaMD, including definitions, classifications, and risk assessment criteria.
Explains how to differentiate between regulated and unregulated software, particularly focusing on intended use.
- AI and Machine Learning Guidance
The FDA emphasizes the importance of transparency and validation for AI-based products.
Developers are encouraged to document the algorithms, training data, and decision-making processes to ensure explainability and trustworthiness.
- Clinical Decision Support (CDS) Guidance
Specifies when CDS tools are regulated. Key criteria include whether the tool provides recommendations that a clinician can independently verify and override.
Encourages developers to design explainable AI that allows healthcare professionals to understand and evaluate the decision-making process.
- Digital Health Policy Navigator
An online questionnaire that helps developers determine whether their product qualifies as a medical device and which guidance documents apply.
Conclusion
Developing SaMD involves navigating stringent standards and managing unique challenges, from mitigating software risks to ensuring compliance with global benchmarks. By adopting robust methodologies and aligning development practices with IEC 62304 and IEC 82304-1, developers can create safer, more effective medical devices that transform healthcare.
Developing Software as a Medical Device (SaMD) requires more than just technical know-how — it demands a deep understanding of compliance, risk management, usability, and lifecycle documentation. As SaMD continues to reshape healthcare delivery, the need for structured, secure, and scalable development practices becomes even more critical.
By aligning with international standards, integrating security and usability from day one, and adopting agile methodologies responsibly, SaMD developers can build innovative products that are not only functional but also trusted by regulators and users alike.
Whether you’re launching your first SaMD solution or optimizing an existing one, the right development strategy will ensure your product is safe, compliant, and ready for global success.
5 Key Takeaways for SaMD Developers
- Embrace Global Standards Like IEC 62304
Standards like IEC 62304 and IEC 82304-1 provide the regulatory foundation for developing compliant, safe, and high-quality medical software. Don’t treat them as checkboxes — integrate them into your everyday processes. - Integrate Risk Management Early
Risk isn’t just an afterthought — it’s a continuous process. Use frameworks like ISO 14971 and the P1/P2 model to identify, mitigate, and monitor potential hazards throughout the software lifecycle. - Use Agile, But Stay Compliant
Agile methods like Scrum and iterative development can be harmonized with regulatory requirements when paired with robust documentation, sprint reviews, and traceability logs. - Prioritize Usability and Security
Standards like IEC 62366-1 (usability) and IEC 81001-5-1 (cybersecurity) are not optional. Build intuitive, secure products that protect users and reduce liability risks. - Leverage Expert Partners Like Gart
SaMD development is complex — partnering with domain experts like Gart Solutions ensures you’re not navigating it alone. From cloud migrations to regulatory audits, expert guidance accelerates your path to market.
See how we can help to overcome your challenges









